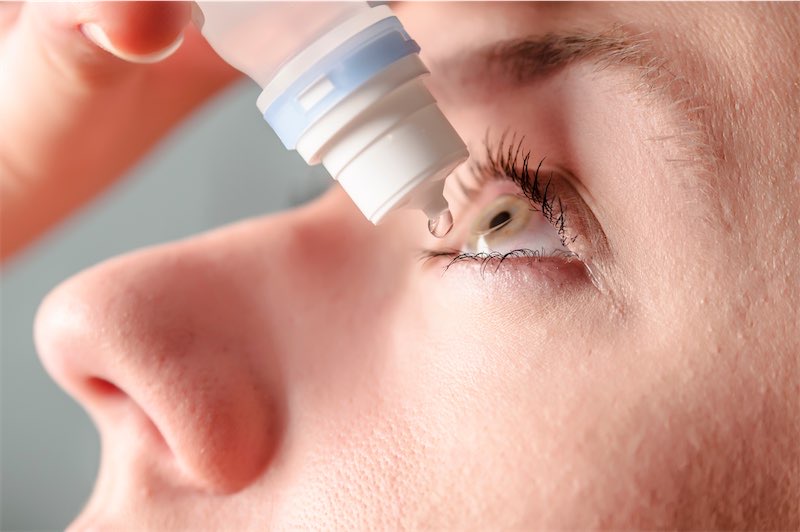
EzriCare Artificial Tears Lawsuit
February 10, 2023
Cryptocurrency Exchange Frozen Account & Crypto Theft Claims
April 4, 2023
EzriCare Artificial Tears Lawsuit
February 10, 2023
Cryptocurrency Exchange Frozen Account & Crypto Theft Claims
April 4, 2023
Elmiron is a prescription medication that has been used to treat interstitial cystitis, a persistent bladder ailment that affects millions of people worldwide. In recent years, however, the medicine has been associated with a rare but serious adverse effect: vision loss and blindness. Patients who have experienced this permanent and life-altering injury are now filing Elmiron-related claims.
In 1996, the U.S. Food and Drug Administration (FDA) authorized Elmiron, also known as pentosan polysulfate sodium. Interstitial cystitis is a severe and frequently debilitating bladder ailment that causes urine urgency, frequency, and pain. Elmiron reduces inflammation and pain by building a protective coating on the bladder’s inner lining.
In recent years, multiple studies have connected the long-term use of Elmiron to pigmentary maculopathy, an uncommon but dangerous eye disorder. The macula, the core portion of the retina responsible for sharp, clear vision, degenerates in this disorder. If the illness worsens, it can cause severe vision loss and perhaps blindness.
Often, hazy vision and reading difficulties are the initial warning indications of Elmiron-related vision disorders. When the illness progresses, individuals may have dark spots or blind areas in their central vision, making it difficult for them to discern faces, read, drive, or conduct other common tasks. Unfortunately, pigmentary maculopathy has no known treatment, and discontinuing Elmiron does not restore the harm.
Patients who have taken Elmiron and suffering visual loss are filing lawsuits against the drug’s producers, Janssen Pharmaceuticals and Johnson & Johnson, due to these severe adverse effects. These claims argue that the drug firms failed to sufficiently warn patients and physicians about the hazards associated with long-term Elmiron usage, and that they continued to market and sell the drug while being aware of the risk of eyesight loss.
In response to the increasing number of Elmiron cases, Janssen and Johnson & Johnson have published a statement admitting the risk of eye injury and suggesting that patients who take the drug have routine eye examinations. Yet, many patients and their families claim that this warning came too late and that the medication producers should have done more to warn about the risks and prevent the occurrence of these severe side effects.
If you or a loved one has taken Elmiron and had vision loss or other eye problems, you should seek immediate medical assistance and see a lawyer who can explain your legal rights and choices. A successful lawsuit can help you receive compensation for medical expenses, lost wages, and other losses, as well as hold the drug corporations accountable for their acts.

