Helping Hundreds of Families Nationwide
Across the country, many families have filed lawsuits against manufacturers of Similac and Enfamil baby formulas, seeking compensation for the trauma their families have suffered due to a diagnosis of necrotizing enterocolitis (NEC).
Why Are Parents Filing Baby Formula Lawsuits?
Plaintiffs in the baby formula lawsuits allege that companies such as Abbott Laboratories and Mead Johnson Nutrition have long been aware of the increased risk of premature infants developing NEC when fed a cow’s milk-based formula but have failed to place warning labels on their products. As a result, thousands of infants and their families have suffered.
NEC is a serious and often deadly gastrointestinal injury that can occur when premature infants are fed certain brands of baby formula in the hospital, including Similac and Enfamil. Cow’s milk-based formulas greatly increase the risk of infants contracting NEC.
Most cases are currently pending in Madison County, Illinois, with anticipated consolidation in the Connecticut District Court by federal courts. Mr. Selinger is reviewing cases where victims were born as far back as 2001 and believes that some cases have values exceeding millions of dollars.
Premature infants can develop NEC when harmful bacteria rupture the walls of the intestines. These sections of the intestine often become inflamed. If left untreated, these inflamed sections often die. As the tissue dies, there is a high risk that the bacteria will leak into the abdomen or infiltrate the bloodstream.
Call Today for a Free Case Evaluation:
888-NEC-MILK
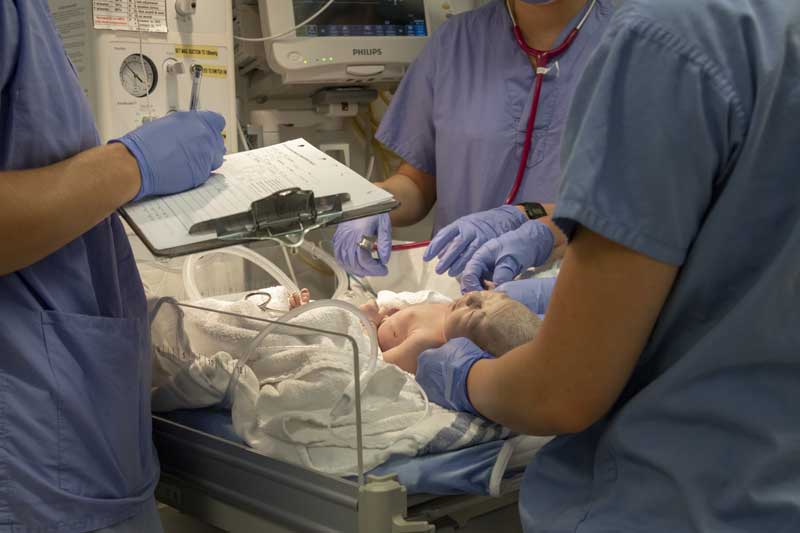
Premature infants can develop NEC when harmful bacteria ruptures the walls of the intestines. These sections of the intestine often become inflamed and if left untreated these inflamed sections often die. As the tissues die there is a high risk that the bacteria will leak into the abdomen and / or infiltrate the bloodstream.
NEC often has a progressive timeline. The beginning stages of mild feeding problems to systemic sepsis infection is often short.
If treated too late, other life-altering conditions include:
- Narrowing of the intestines
- Short bowel syndrome
- Developmental delays
In most cases, a premature infant diagnosed with NEC will require surgery to remove the dead or dying portions of their intestines and repair any resulting holes through an ostomy procedure. While surgery can save lives, it can also result in lifelong injuries or disabilities. Unfortunately, 30% of preemies who undergo surgery do not survive.
These formula makers have a duty to properly research and disclose potential risks so that doctors and parents can make informed decisions about to what their infants. Allegations raised against Enfamil and Similac include:
- Cow’s milk-based formula greatly increase the risk for premature infants to develop NEC.
- The warning labels on the formula are egregiously broad and fail to reasonably disclose the risks of NEC, potential surgery outcomes, and the risk of death for preemies.
- These companies have poured billions of dollars into marketing that has targeted mothers to discourage them from breastfeeding.
- These products were misleadingly introduced on the market as derivatives of breast milk, when, in fact, they were cow’s milk-based products.
- Despite known NEC risks surrounding cow’s milk-based formulas, Enfamil and Similac were sold to parents and hospitals for years, generating large profits at the expense of infants’ lives.
Which Baby Formulas are Being Listed in the Lawsuits?
Abbott Laboratories makes Similac. The following Similac products are cow’s milk-based:
- Similac NeoSure
- Similac Alimentum Expert Care
- Similac Liquid Protein Fortifier
- Similac Human Milk Fortifier
- Similac Human Milk Fortifier Hydrolyzed Protein Concentrated Liquid
- Similac Special Care 20
- Similac Special Care 24
- Similac Special Care 24 High Protein
- Similac Special Care 30
Mead Johnson Nutrition also makes a cow’s milk-based formula called Enfamil, which includes the following formulas:
- Enfamil NeoPro EnfaCare Infant Formula (Mead Johnson advertises this formula as the "best" for premature babies)
- Enfamil Premature Infant Formula 20 Cal with Iron
- Enfamil 24 Cal Infant Formula
- Enfamil Premature Infant Formula 24 Cal High Protein
- Enfamil Premature Infant Formula 24 Cal with Iron
- Enfamil Premature Infant Formula 30 Cal with Iron
- Enfamil Human Milk Fortifier
Isn’t A Human Milk Fortifier Made From Human Milk?
No. Despite the name, human milk fortifiers are not made from human breast milk. Instead, they are made from cow’s milk. Some of the current lawsuits allege that Abbott Laboratories and Mead Johnson Nutrition use this name to deliberately mislead consumers into believing that these are safe, human milk-based products.
Have Any Similac Or Enfamil Formulas Been Recalled?
No, there have been no recalls of Similac or Enfamil baby formulas. This, however, doesn’t mean that the products aren’t causing harm to premature babies.
As the scientific community conducts further research and legal claims are reviewed, additional baby formula brands may be included in the ongoing litigation.
What Is Necrotizing Enterocolitis (NEC)?
Necrotizing enterocolitis (NEC) is a disease that affects the gastrointestinal system that is most commonly seen in premature babies. NEC develops when tissue in the small or large intestine is injured or inflamed, which can cause the tissue to die. In some cases, a perforation (hole) in the intestine occurs, spilling bacteria into the abdomen. This bacterium can then enter the bloodstream and cause sepsis.
In some cases, a perforation, or hole in the intestine occurs, spilling bacteria into the abdomen. This bacteria can then enter the bloodstream and cause sepsis.
What Are The Symptoms Of NEC?
A baby who has developed NEC could have any of the following symptoms:
- Abdominal Swelling
- Lethargy
- Vomiting (pale green liquid)
- Diarrhea
- Constipation
- Hypotension (a low blood pressure)
- Bradycardia (low heart rate)
- Low Temperature
Babies who have NEC typically have difficulty feeding.
How Is NEC Diagnosed?
When a doctor suspects NEC, they order diagnostic testing such as x-rays, ultrasound, and bloodwork.
How Is NEC Treated?
It’s important to treat NEC as soon as it is diagnosed. First, all feedings are stopped. Then, one or more of the following treatments may take place:
- Nasal or oral tubes placed into the stomach to release trapped gas and fluids
- IV fluids
- Antibiotics
- X-rays at regular intervals to monitor changes to the abdomen
- Oxygen therapy
- Surgery
If a preterm infant is too small to undergo a surgical procedure, a catheter will be placed in their abdomen to relieve pressure and drain fluids.
Surgery For NEC
Although not all babies with NEC need surgery, one in four patients do. During the procedure, the pediatric surgeon will inspect the intestine and the abdomen. If part of the intestinal tissue has died, that tissue will be removed and the healthy tissue sewn together in a procedure known as an anastomosis.
In some cases, an ostomy must be performed. During this procedure, a section of the healthy intestine is joined with a stoma, or opening in the abdomen, so that stool can continue to exit the body. A second surgery will be needed to rejoin the intestine once the danger has passed.
Often, infants who undergo NEC surgery have complications that require additional surgical procedures to fix.
Can NEC Cause Permanent Damage?
Yes. While many babies fully recover and have no lasting damage, others aren’t as lucky. The three most common complications caused by NEC are intestinal stricture, cholestatic liver disease, and short bowel syndrome.
Intestinal Stricture
A stricture is a narrowing of the intestines, typically caused by scar tissue from ongoing inflammation, such as that from NEC. The most common locations for strictures are the ileum (the last part of the small intestine) and ileocecal valve (the entrance to the large intestine from the small intestine). In severe cases, strictures can cause intestinal blockage, which may require surgery to repair.
In extreme cases, the intestinal stricture can cause a blockage in the intestine. Like NEC, this blockage may require surgery to repair.
Cholestatic Liver Disease
Cholestatic liver disease is a condition where the liver is inflamed due to an accumulation of bile acids caused by impaired bile formation or flow. In preterm infants, this disease is caused by bacteria as well as parenteral feeding (when liquid nutrition is delivered via IV), which is common in low-weight preterm infants and those with NEC.
Treatment requires stopping the cause of inflammation, such as parenteral feeding, but this may not be possible for infants with NEC who cannot feed on their own. Adding certain lipids to the diet may help keep the liver healthy. If left untreated, cholestatic liver disease can lead to cirrhosis and liver failure.
Short Bowel Syndrome
Short bowel syndrome is caused by the removal of a large section of the small intestine due to tissue death, which results in the body being unable to take in required nutrients. Patients with this syndrome require special diets, supplements, and may need a port for nutrient delivery to prevent malnutrition. Complications from this condition are common and can be severe, and transplant surgery may be necessary in some cases.
Patients with short bowel syndrome require special diets, supplements, and may need a port through which nutrients can be delivered to avoid malnutrition.
Complications from this syndrome are common and can be severe. In some cases, a transplant surgery may be required.
In addition to intestinal stricture, liver injuries, and short bowel syndrome, infants with NEC are at risk of experiencing growth and developmental delays.
What Causes NEC?
According to the National Institute of Health, the medical community has yet to clearly identify all the possible causes of NEC in premature babies. However, decades of research has shown that there is a clear link between formulas made with cow milk and an increased risk of developing NEC.
Scientific Research Links Cow’s Milk-Based Baby Formulas to NEC
Over the last several decades, the scientific community has performed dozens of studies attempting to identify the causes of NEC. Conducted by a range of reputable medical organizations ranging from the Office of the Surgeon General to the American Academy of Pediatrics, many of these studies have shown a link between cow’s milk-based formulas and an increased risk of infants developing NEC.
The Lancelet - “Breast Milk and Neonatal Necrotizing Enterocolitis”
In 1990, a study was published in The Lancelet, examining a total of 926 preterm infants. Of those, a total of 51 developed NEC. The study reviewed the feeding plan of each infant and ultimately found that:
- Preterm infants who were exclusively fed formula diets were 6-10 times more likely to be diagnosed with NEC than those who were fed with breast milk.
- Preterm infants who were exclusively fed formula diets were 3 times more likely to develop NEC when compared to those who were fed a combination of formula and breast milk.
The study estimated that in neonatal units that used formula only diets, an additional 500 infants would be diagnosed with NEC each year and that an additional 100 would not survive the complications caused by NEC.
Journal Of Pediatrics - “Human Milk Fortification Increases Bnip3 Expression Associated With Intestinal Cell Death In Vitro”
This study, performed in 2010, was specifically designed to determine how a cows’ milk formula impacted intestinal cells vs human breast milk.
Cultured intestinal cells were exposed to a bovine-based human breast milk fortifier and cell death, intracellular oxidation, and cell damage were measured after exposure. It was found that exposure to the bovine-based human breast milk fortifier greatly increased cell damage and cell death when compared to exposure to saline and other controls.
The results of the study showed that intestinal cells exposed to human breast milk were 90% less likely to trigger cell death. This means there is a 90% less chance of inflammation and infection that causes NEC.
Office of the Surgeon General
In 2011, the Office of the Surgeon General in the United States, in conjunction with the Office of Women’s Health, and the Centers for Disease Control released a statement urging society at large to support breastfeeding. A statistic was included, stating that premature infants who are not fed with human breast milk are 138% more likely to develop NEC.
Journal Of The American Academy Of Pediatrics
Published in 2012, this study reviewed the data of four different clinical trials performed between 1983 and 2005. The conclusion was that preterm infants fed exclusively breast milk had a 58% reduction in the instance of NEC. It was also noted that a separate study showed a 77% reduction in NEC when infants were fed breast milk instead of cows’ milk formulas.
Expert Review Of Clinical Immunology
This is a 2014 review of the evidence that existed at that time linking cows’ milk formulas to NEC. In the review, it was noted that … ”in premature infants who were greater than 30 weeks gestation, NEC was 20 times more common in formula fed infants versus breast fed infants.”
Pediatria
Titled “In time: human milk is the feeding strategy to prevent necrotizing enterocolitis”, this 2015 study out of Portugal found that a human breast milk diet greatly reduced a preterm infant’s chances of developing NEC, urinary tract infections, and late-onset sepsis. The study also showed that infants fed breast milk had shorter hospital stays.
Breastfeeding Machine
“Beyond Necrotizing Enterocolitis Prevention: Improving Outcomes with an Exclusive Human Milk-Based Diet”, noted a greatly decreased risk of developing NEC in infants who were fed breast milk.
While all of the factors that could contribute to a preterm infant have yet to be identified, the overwhelming evidence shows a clear link between cows’ milk formula and the risk of developing NEC.
It is this overwhelming evidence that has caused many parents to file product liability lawsuits against baby formula manufacturing companies.
What Is A Product Liability Lawsuit?
Under product liability laws, a product is required to meet the ordinary expectations of a consumer. If it fails to do so, the consumer has the right to take legal action against those responsible in the form of a product liability lawsuit.
A product liability lawsuit is a type of personal injury lawsuit. This type of legal claim is filed by plaintiffs who allege that a product has caused them serious harm.
Product liability lawsuits are typically broken down into three categories: products that have defects that occurred during the design process, products that have defects that occurred during manufacturing, and products that did not include an adequate warning label.
Who Is Eligible To File A Baby Formula Lawsuit?
Our experienced legal team is reviewing Similac and Enfamil cases for families who have children who were:
- Born Prematurely
- Were Fed Similac or Enfamil Formulas
- Diagnosed With Necrotizing Enterocolitis
If you or a loved one believe that you may have a case, don’t hesitate to contact our legal team.
Examples of Similac and Enfamil Cases
Here are a few examples of the cases that have recently been filed against Abbott Laboratories and Mead Johnson Nutrition:
Alicia Restad et al. v. Abbott Laboratories & Mead Johnson Nutrition Company
This case was filed in the Eastern District of California on May 14th, 2021 by Alicia Restand on behalf of her son, Daniel Renteria-Hernandez.
Daniel was born on April 29th, 2019, in Merced, California. He was born prematurely at 31 weeks and weighed only 2 lbs. and 2 ounces. He was immediately admitted to the NICU, where during his stay, he was fed formula manufactured by Abbott Laboratories and Mead Johnson Nutrition. After rapidly developing NEC, Daniel passed away on May 15th, 2019, at just 16 days old.
Daniel quickly developed NEC. He died on May 15th, 2019, having lived only 16 days.
His parents allege that not only did Abbott Laboratories fail to warn doctors and parents about the dangers of feeding cow’s milk-based formulas to premature babies, but they also, as recently as 2016, actively marketed seven of their products as suitable for “Premature/Low birth-Weight Infants”. The seven products included Liquid Protein Fortifier, Similac® NeoSure®, Similac® Human Milk Fortifiers, Similac® Special Care® 20, Similac® Special Care® 24, Similac® Special Care® 24 High Protein, and Similac® Special Care® 30.
Shannon Hall, et al. v. Abbott Laboratories,
Filed in the Northern District of Illinois on January 5th, 2022, the plaintiff is a woman whose infant son, Eli, passed away from complications caused by NEC.
Born prematurely on November 27th, 2019, in Winston Salem, North Carolina, Eli was admitted into the NICU for care and observation. For the first week of his life, Eli was fed breast milk that was provided by his mother and other donors. On December 9th, a human milk fortifier was added to his diet.
On December 20th, the decision was made to add Similac Special care, a cow’s milk-based formula, to Eli's diet. He was fed this formula in combination with breast milk and the human milk fortifier every three to four hours until January 6th.
On January 6th, Eli was noted to have abdominal distension and other symptoms of NEC. Despite being evaluated by a pediatric surgeon, he wasn't diagnosed with Stage III NEC until the next day. At that time, he was rushed into emergency surgery, but the actions taken by the medical team were too late. Eli passed away on January 7th, 2020.
DaNitra Rhodes, et al. v. Abbott Laboratories
DaNitra Rhodes filed a lawsuit on January 14th, 2022, in the District Court of Illinois on behalf of her infant son, Kingston Grisby.
Kingston was born on April 19th, 2015, in Dallas, Texas. He was born premature at 26 weeks and admitted into the NICU. His mother was able to successfully pump her own breast milk and Kingston was on a breast milk-only diet until May 14th, 2015. Human milk fortifier was added to his diet to increase his caloric intake to 22 calories an ounce.
On May 22nd, 2015, Similac NeoSure was added to Kingston’s diet. By May 28th, he had several episodes of repeated vomiting and developed abdominal distension, a common sign of NEC. He was diagnosed with the disorder the same day and was rushed into surgery where the surgeon was forced to remove half of his small intestine and performed an ostomy. It wasn’t until July 7th that surgeons were able to perform an anastomosis, rejoining his interstitial tract.
Several weeks later, a third surgery was performed to treat complications from the first two surgeries. Today, Kingston has been diagnosed with short bowel syndrome and as a result, will require ongoing medical care for the rest of his life.
Crawford v. Abbott Laboratories, Inc.
Filed in the Southern District of Florida, the plaintiff, Candace Crawford, gave birth to her daughter, ZaRiyah, at 34 weeks on December 2nd, 2019. ZaRiyah was only 3 lbs. and 14 ounces. The doctors made it known that rapid weight gain was important for ZaRiyah.
At first, ZaRiyah was fed breast milk from her mother and other donors. Then, after three days, cow’s milk-based formula was added to her diet. It only took a few days for her to develop the symptoms of NEC, including a lack of appetite and a firm abdomen. On December 9th, 2019, she was taken to surgery where it was determined that none of her bowel was salvageable.
Her parents were forced to make the decision to withdraw life-saving efforts and to provide comfort and care until she passed on December 11th, 2019.
These are just a few examples of the heart-wrenching situations that might have been avoided if companies like Abbott Laboratories and Mead Johnson Nutrition had adequately warned consumers about the risks of feeding their formulas to premature babies.
What Must be Proven to Win a Similac or Enfamil Lawsuit?
When a product liability lawsuit is filed, the plaintiff will need to prove the following in order to win their case:
The Product Listed In The Lawsuit Is Defective Or Dangerous
Plaintiffs must prove that the manufacturing company should have warned the medical community and parents about the increased risk of a premature baby developing necrotizing enterocolitis when fed one of their cow’s milk-based formulas.
The Plaintiff Was Injured And Suffered Losses
When a plaintiff files a product liability lawsuit, they are seeking compensation for losses that were sustained, known as damages. Damages are broken down into two categories: compensatory and punitive.
Compensatory Damages
Compensatory damages seek to make a plaintiff whole. While this might not be physically possible, these damages are meant to provide the plaintiff with the money needed to pay for the losses directly sustained by using a defective product. These damages are split into two types:
- Economic losses: This refers to money that a plaintiff had to pay or lost out on making because of the illness or injury that was sustained. Economic losses may include medical expenses and lost wages or profits (both past and future).
- Non-economic losses: These cover losses that are difficult to quantify, such as pain and suffering.
Punitive Damages
Punitive damages are issued when a court is seeking to punish a defendant to prevent them from behaving in a similar manner in the future for fear of monetary losses.
Punitive damages are not awarded in every case. However, there have been many product liability cases where punitive damages were awarded to plaintiffs. For example, Johnson & Johnson was ordered to pay $186.5 million in punitive damages to plaintiffs who filed talcum powder lawsuits.
The Plaintiff Used The Product As Was Intended
In order to win a product liability case, a plaintiff has to show that the product was used as intended by the manufacturer.
How Quickly Must a Product Liability Lawsuit be Filed?
In all 50 states, there is a limit to the amount of time a plaintiff has to file a product liability lawsuit, known as the statute of limitations. For example, in New York, a person injured by a dangerous product has the right to file a lawsuit within three years. This three-year limit begins when a person discovers, or should have reasonably discovered, that the product caused them harm.
The three-year limit begins when a person discovers or should have reasonably discovered, that the product caused them harm.
How Many Similac and Enfamil Lawsuits Have Been Filed?
More than 50 Similac and Enfamil lawsuits were filed in 2021 and new cases are being filed each day. In fact, a motion was recently filed asking the Supreme Court of Illinois to consolidate all pending NEC infant formula cases into a single case in Madison County. If granted, this would mean a multidistrict litigation (MDL) would be formed.
What Is Multidistrict Litigation?
Multidistrict Litigation is a legal procedure that allows plaintiffs from throughout the country to have their claims transferred to one court. One judge will oversee the entire process, including the pretrial and discovery process.
Why Are MDLs Formed?
MDLs can be beneficial for plaintiffs and the court system. Plaintiffs benefit because their attorneys can pool resources and coordinate efforts. An MDL benefits the court system by moving all complaints into one court and ensuring that the lawsuits proceed in a timely and orderly fashion, which helps reduce the backlog.
Each year, around 15% of all civil lawsuits are consolidated in this fashion.
Who Approves The Formation Of An MDL?
The Judicial Panel on Multidistrict Litigation (JPML) is a panel of seven judges that reviews motions to form an MDL.
The JPML reviews three factors when considering a motion to form an MDL:
- Do the cases involved have one or more similar questions of fact? A question of fact is the question that must be resolved by a judge and/or jury. It is the main issue in the cases.
- Will a transfer to one court promote efficiency and fairness to all of the involved parties?
- Will the creation of an MDL be beneficial to all involved parties?
If a majority of judges on the JPML determine that the cases should be consolidated, a transfer order is initiated. This transfer order will take the lawsuits from the federal court they were initially filed in and send them to a single federal district court. This district court is called the “transferee court”.
When choosing a transferee court, the Panel will review answers to questions like:
- Where are all parties located?
- How difficult is it to travel to the courthouse for those who aren’t local?
- Where was the product manufactured?
- Where was the first lawsuit filed?
- Where are the majority of lawsuits pending?
- Do any courts have specialized experience or knowledge pertaining to these cases?
- How full is the potential court’s docket?
- What courtroom technology is available?
The majority of Similac and Enfamil lawsuits have been filed in the state of Illinois, which is why the motion to consolidate was filed in that state.
It can take months for an official ruling on a motion to form an MDL.
What Happens After An MDL Is Formed?
The Plaintiff Steering Committee is comprised of attorneys who are appointed to oversee the interests of all plaintiffs involved in the MDL. These attorneys are appointed based on their knowledge of the case, the number of clients they represent, and their ability to perform the required work during the discovery process.
The Plaintiff Steering Committee is formed of attorneys who are appointed to oversee the interests of all plaintiffs involved in the MDL. Attorneys are appointed based on their knowledge of the cases, the number of clients they represent, and their ability to perform the required work during the discovery process.
What Is Discovery?
Discovery is the time during a lawsuit when both sides exchange information about the evidence that will be presented at trial and the witnesses that may take the stand. Discovery consists of four actions:
- Interrogatories: Interrogatories are written questions from one party to another. These questions are intended to gather facts about the case, including witness information, medical history, and a description of the losses that were sustained.
- Request For Production: This is when a formal written request is made for specific documents that may be relevant to the case. This might include copies of medical records, insurance paperwork, photographs, and receipts. All parties are required to produce relevant information when a request is made.
- Request For Admission: In a request for admission, one party will present a factual statement to the other. The receiving party must admit, deny, or object to the factual statement.
- Deposition: A deposition is an out-of-court interview that is recorded by a court transcriber. A transcript of the deposition may be used later in court to corroborate or dispute testimony.
How Long Does The Discovery Process Take?
A deadline for discovery will be set by the judge; however, this process can take months.
Once completed, the judge will set dates for several bellwether trials.
MDL Bellwether Trials
Bellwether trials are essentially test cases for those involved in an MDL. These trials help to give the plaintiffs an idea of how the majority of cases will be decided and what type of compensation that plaintiffs should receive.
Bellwether trials often result in settlement agreements.
Can A Defendant Fight Against The Formation Of An MDL?
Yes, in some cases, defendants do not want an MDL to be formed. They have the right to file motions and argue against the formation. At this time, the JPML has motions by defendants Abbott Laboratories and Mead Johnson Nutrition for an extension of time to respond to the consolidation request.
Can Additional Cases Be Added To An MDL After It Is Formed?
Yes, additional cases can be filed under an MDL. These cases are known as “tag-along” cases.
How Quickly Will Similac and Enfamil Lawsuits be Resolved?
It can take months or even years for a product liability lawsuit to reach a conclusion. Ultimately, there are two outcomes: a settlement is reached between the parties or the case is taken to court and a verdict is rendered.
Cases that are resolved through settlement typically end much sooner than those that wait on a court date.
Is It Better To Accept A Settlement Or Go To Trial?
There are benefits to accepting a settlement offer. After all, it ensures that a plaintiff gets their compensation in a timely manner and receive a quick resolution. However, it’s important to remember that once an offer has been accepted, no additional legal action can be taken.
Unfortunately, not all offers are worth considering. If you receive any offers, be sure to send them to our legal team so we can review them carefully.
How Much are Similac and Enfamil Lawsuits Worth?
These cases have yet to go to trial; however, many NEC medical malpractice lawsuits have. We have reviewed the outcomes from these cases to give you an idea of what the settlements and verdicts from Similac and Enfamil cases could be.
New York NEC Medical Malpractice Lawsuit Results In $1,350,000 Settlement
In this case, a two-day-old infant developed NEC after being fed a combination of breast milk and formula. His parents filed a medical malpractice lawsuit against the hospital, claiming that their child developed the condition after the hospital failed to feed him a breast milk-only diet.
Massachusetts NEC Lawsuit Ends With $7,050,000 Verdict
In this case, a premature baby was transferred to the NICU where her physician ordered her to be fed with formula. She ultimately developed NEC and died from the complications. Her parents filed a lawsuit alleging that the hospital staff failed to diagnose NEC in a timely manner, ultimately delaying treatment that could have prevented her death.
The compensation provided to parents and guardians who file Similac or Enfamil lawsuits will vary and will be based on the losses they sustained.
Could My Family also be Eligible for a Medical Malpractice Lawsuit?
Yes, there are cases where a family could be eligible to file both a product liability lawsuit and a medical malpractice lawsuit.
Medical malpractice lawsuits are filed when a patient suffers harm while under the care of a medical provider. There are several types of medical malpractice:
1. Failure To Diagnose
A failure to diagnose occurs when a doctor fails to take the proper steps to correctly diagnose a medical condition. A failure to diagnose means that a medical condition remains untreated and as a result, could cause damage to a patient that could have been avoided.
2. Misdiagnosis
When a patient is incorrectly diagnosed, they may be prescribed medications and undergo treatments that are unnecessary, while the actual medical condition remains untreated.
3. Surgical Error
Surgeons have a huge responsibility and when they make a mistake, it’s the patient that suffers.
4. Medication Errors
Medications can be wonderful, providing comfort and saving lives. But when given to the wrong patient or in the wrong quantities, a medication can cause irreversible damage.
Birth Injuries
Common birth injuries include brachial palsy, fractures, and cerebral palsy.
During a review of your case, our lawyers can determine if a medical malpractice lawsuit can be pursued against a doctor, nurse, hospital, or other medical provider.
Why Should I Choose Selinger Law Group to Represent My Family?
Selinger Law Group has represented thousands of clients who have been hurt by defective medical devices and pharmaceutical drugs.
John Selinger, Esq. is a former district representative and past Bar President of the New York State Bar Association. Mr. Selinger has been named in the Law Dragons Top 500 list, National Trial Lawyers Top 100, and Super Lawyers 12 years in a row, a distinction awarded to only 5% of personal injury lawyers in the state of New York.
Our team has fought aggressively and won significant compensation for our clients, including a recent $15 million dollar award for a brain injury lawsuit.
Contact us today for a case review and to learn about how we can help you fight for the compensation that you and your child deserve.


