3M Military Earplugs Lawsuit Update
March 8, 2022
Possible link between Tylenol during pregnancy and autism, ADHD in kids, study finds
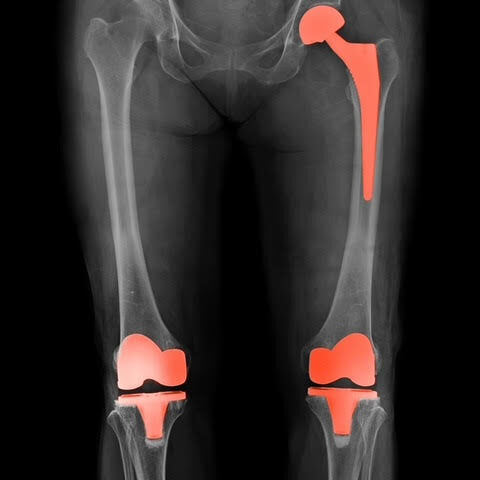
January 30, 2023A recall has been issued for over 140,000 Opetetrak, Optetrak Logic, and Truliant knee replacement systems, 130,000 hip replacement systems, and 1,500 ankle replacement systems.
Exactech, a medical device company that develops and markets orthopaedic implant devices and related surgical instruments, has issued a recall for specific models and lot numbers of the company’s knee, hip, and ankle replacement joints. The recall was issued due to reports of the high failure rate of the devices, with some patients experiencing failure and complications within a year of implantation. According to the FDA, these complications can include pain, swelling, difficulty walking or bearing weight, limited range of motion, instability or looseness in the affected joint, and the need for revision surgery.
According to Exactech, defective bags caused these medical devices to prematurely fail. The apparent defect in these bags could allow oxidation of the parts, which could severely degrade the product and increase the risk that the devices would fail once implanted into a patient’s body. As Exactech has stated in its recall notice and in court filings, the oxidation could cause “accelerated wear debris production and bone loss, and/or component fatigue cracking/fracture, all leading to corrective revision surgery.” This “corrective” revision surgery can be more complicated and painful than original implantation surgery.
Exactech is currently facing multiple lawsuits regarding the alleged failure of its knee replacement devices. Selinger Law Group is actively seeking clients who have been affected by these defective devices. If you or a loved one has received a knee replacement from Exactech and are experiencing complications or problems, we may be able to help you seek compensation for your injuries and damages. Contact us to learn more about your legal options and to schedule a free consultation.
If you had a knee, hip or ankle replacement and are having any of the following symptoms:
- Pain in the knee, hip, or ankle
- Swelling
- Difficulty walking or bearing weight
- Limited range of motion
- Instability or looseness in the affected joint
- Noise or creaking in the affected joint
- The need for revision surgery